
The PHC Floodlight Product Team aims to develop a robust digital tool that tracks disease progression in patients with multiple sclerosis. To this end, FLOODLIGHT is being developed as a smartphone application that captures patient-reported symptoms, and both active and passive measures of neurologic performance. FLOODLIGHT is being evaluated in Roche MS clinical trials and real-world MS cohorts, and data generated will be used to support registration of FL as a Software as Medical Device (SaMD).
DevSci OMNI Biomarker Development is leading the biomarker strategy within the Floodlight MS Product Team, and leading development of the cornerstone evidence generation study for the program (FL MS TONiC Study). We’re building collaborations with academic partners to design quality real-world data studies that capture multi-modal data (digital, biological, imaging, clinical), and leading the strategy for next-generation biomarkers in MS. We’re also coordinating the use of FLOODLIGHT in Roche clinical trials to understand whether digital markers could be used as marker of progression in earliest forms of MS.
FLOODLIGHT is a Smartphone App designed to track progression in patients with multiple sclerosis
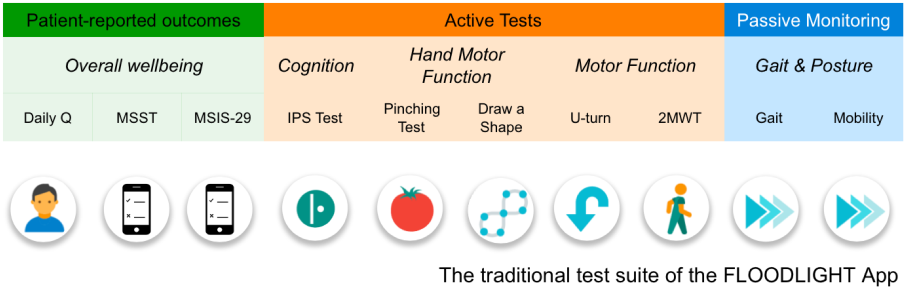
2MWT, two minute walk test; GPS, global positioning system; IPS, information processing speed; MSIS, MS Impact Scale; MSST, MS symptom tracker; PRO, patient reported outcome
Example: Draw a Shape Test captures detailed upper limb function
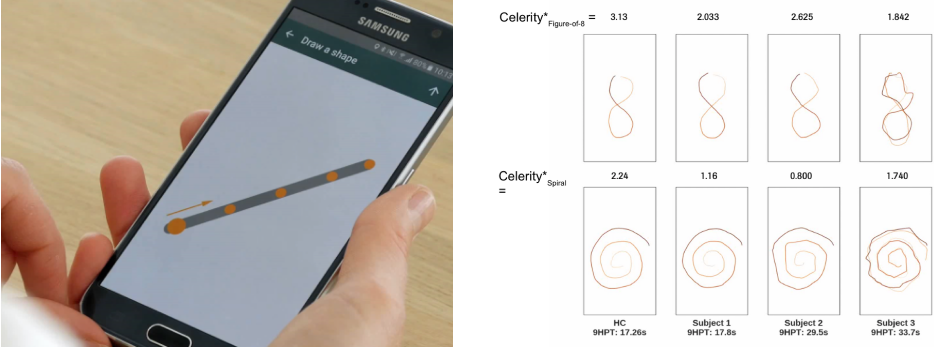
*Celerity, a measure of speed and accuracy: the higher the value the better the performance.
NB: Images included are only intended as illustrative examples from Roche digital outcomes studies. The best of 2 attempts is used.
9HPT, 9-Hole Peg Test; HC, healthy control. Roche data on file
Draw a Shape test discriminates patients with MS from healthy controls

P. Mulero, L. Midaglia, X. Montalban, J. Graves, S.L. Hauser, L. Julian, M. Baker, J. Schadrack, C. Gossens, A. Scotland, F. Lipsmeier, A. Creagh, C. Bernasconi, S. Belachew, M. Lindemann. 2017 ECTRIMS-ACTRIMS Meeting, Poster P1226,
PHC Floodlight real-world MS cohorts
FLOODLIGHT MS TONiC: The cornerstone evidence generation study for FL MS.

The FL MS TONiC study (MN43238) is a collaboration with UK investigators at the Walton Centre and University of Liverpool to deploy FL MS in a large, real world MS population. The study will investigate FL MS in n=1000 patients (700 RMS, 300 PMS) recruited through the ongoing Trajectories of Outcomes in Neurologic Conditions (TONiC) study, and will prospectively collect clinical outcome measures, comprehensive patient-reported outcomes (PROs), genotype and genome sequence data, brain and spinal cord MRI, blood biomarkers, and transcriptomics of peripheral blood mononuclear cells (PBMCs). It aims to investigate the role of FL MS in capturing MS disease trajectory, and to link digital outcomes with meaningful clinical and biologic measures in MS.
Biomarker strategy in FLOODLIGHT real-world MS studies
