
1 minute read
Optimal long-term outcomes in neovascular Age-related Macular Degeneration (nAMD) require frequent injections and patient monitoring as often as once a month, which places a high burden on patients, their caregivers, and health care providers. Clinical studies indicate that the frequency of treatment with anti-VEGF agents required to maintain vision gains is variable between patients. In clinical practice, many patients are treated less frequently than in clinical trials and, as a consequence, experience suboptimal efficacy (Holz et al. 2015; Maguire et al. 2016; Egan et al. 2017).
Ranibizumab is an antibody fragment (Fab) which targets vascular endothelial growth factor (VEGF). Intravitreal injections of ranibizumab (LUCENTIS) were first approved in 2006 for nAMD and then subsequently other ocular indications including retinal vein occlusion, diabetic eye disease, and myopic choroidal neovascularization. The Port Delivery System with ranibizumab (PDS), an innovative delivery system that includes a refillable implant for continuous delivery of ranibizumab into the vitreous, has been investigated in nAMD patients in a Phase 3 trial and has been recently filed, FDA review expected in October 2021.
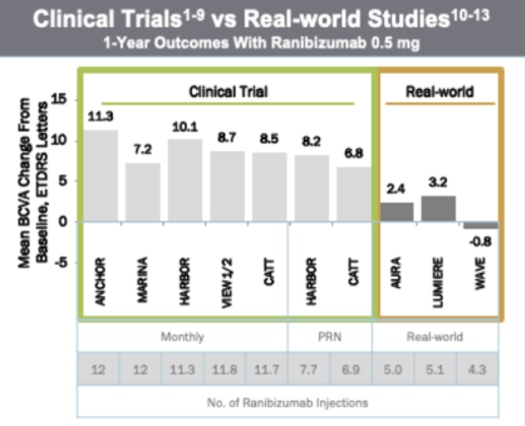
Real-world vision outcomes are non-optimal in part due to the burden of frequent treatment and monitoring. To reliably assess which patients could go longer between injections or between refills with the PDS would enable reduced treatment burden and improved real-world outcomes. Enabling a longer time between refill in those patients that can maintain visual gains with longer intervals would help deliver the maximal value of PDS to patients.
 Back to top
Back to top